April 5, 2019
Dear Valued Phytoextractum Customers,
We know many of you have seen the FDA’s most recent statement on kratom, regarding what they’re calling high levels of heavy metals found in kratom products. At Phyto, we hold the health and safety of our customers as our highest priority, and we take our responsibility to provide pure, safe products seriously. Because of this commitment to our customers, we are constantly working to stay current with Good Manufacturing Practices, and we recently underwent a third-party audit to validate our GMP compliance.
We’d like to address a few things in the FDA’s statement:
First, it’s troubling that we have never been contacted by the FDA about high levels of metals in our products. We are registered as a dry food facility with the FDA, which led us to believe that it was incumbent upon them to alert us to any concerns regarding the safety of our products.
Phytoextractum has no information regarding the samples the FDA used in their tests, including when or how the FDA acquired our products or when they tested the samples they obtained.
We can only assume that they were part of samples taken and tested as part of an FDA investigation of an unrelated food safety issue in March of 2018. FDA representatives were at our facility for several days, inspecting, taking samples, and meeting with our company. They never mentioned an issue with metals at that time.
If these samples are from that investigation, their product lots were pulled from the market over 12 months ago as part of the extensive recall we did in cooperation with the FDA. Further, it would indicate that the FDA was rehashing old information, not in an attempt to protect the public, but as a way to target and further stigmatize kratom.
Additionally, the numbers the FDA is publicizing are misleading. While their recommendations for the presence of heavy metals use the ‘micrograms per kilogram’ format, their recently publicized metal levels are represented in the ‘nanograms per gram’ format.
The FDA is not changing the numbers, i.e. the analytical data, but they are changing how the numbers are presented and therefore interpreted in order to draw more attention to this issue than it actually warrants. If this were an actual threat to public health, we expect the FDA would have moved more quickly to alert vendors and consumers about specific affected products.
While Phyto has always prioritized customer safety, in the year since our product recall we have fine-tuned our policies and procedures to achieve compliance with Good Manufacturing Practices as defined by the FDA and to become an American Kratom Association GMP Qualified vendor. This includes comprehensive lab testing for identity, purity, and contaminants such as high levels of heavy metals for all products.
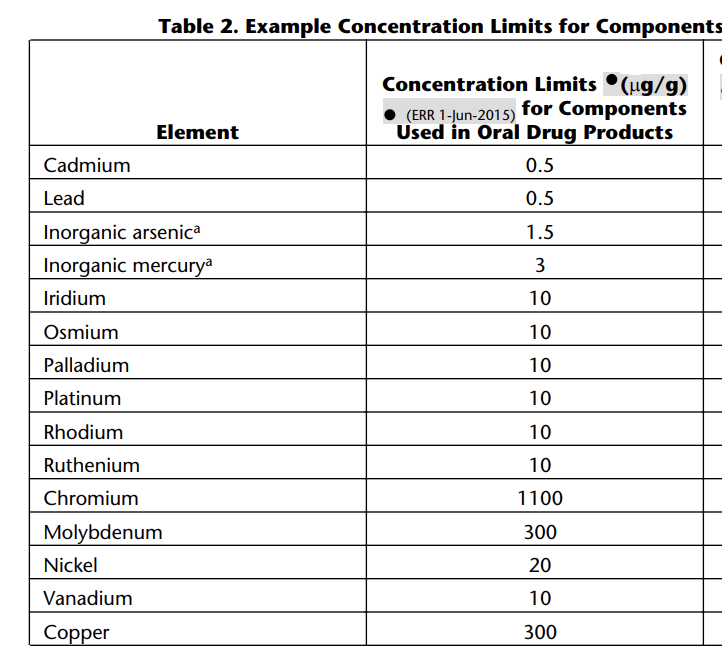
After learning of the FDA’s statement on heavy metals in kratom products, we consulted our testing laboratory. They reviewed our test results and provided the following information: “all metals analyzed in here are at less than limits concentrations assuming that daily [serving] is 10 g. For example, Lead result is 0.450 mg/Kg with the limit of 0.5 mg/Kg. So, it is a PASS result based on USP limits [see chart below].“
We never introduce products to the market that do not pass rigorous quality assurance tests and meet our own high standards. Our company keeps Certificates of Analysis on file for all products, we and are happy to share those with our customers at their request.
Please feel free to contact our customer support team with any questions you may have about our products or practices.